
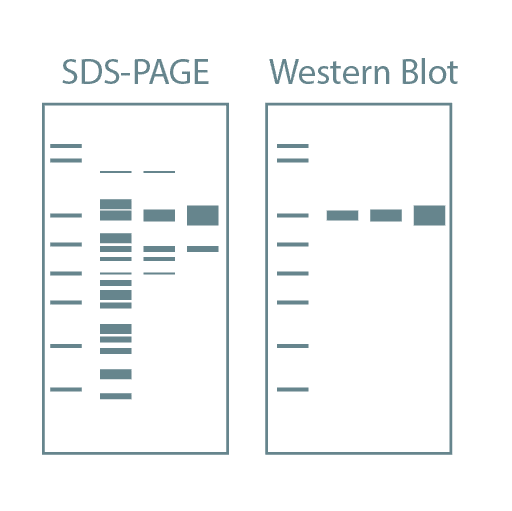
We provide Biochemical Services for protein expression, purification, quality control, and interaction studies. These studies often follow computational services related to protein design and engineering. Our Biochemical Services are optimized for high-throughput execution, enabling us to analyze over 400 samples per month, from expression to purification to initial biophysical characterization (stability, activity).
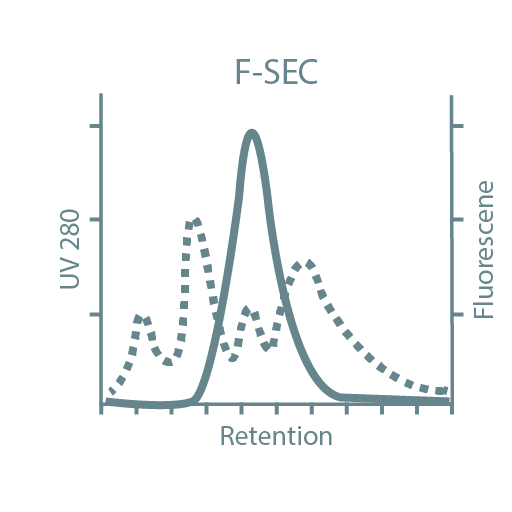
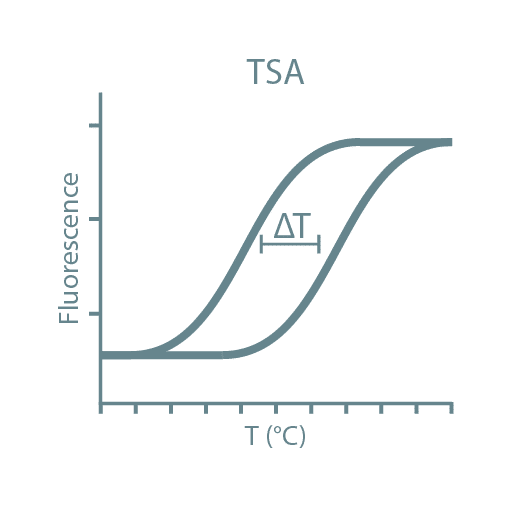
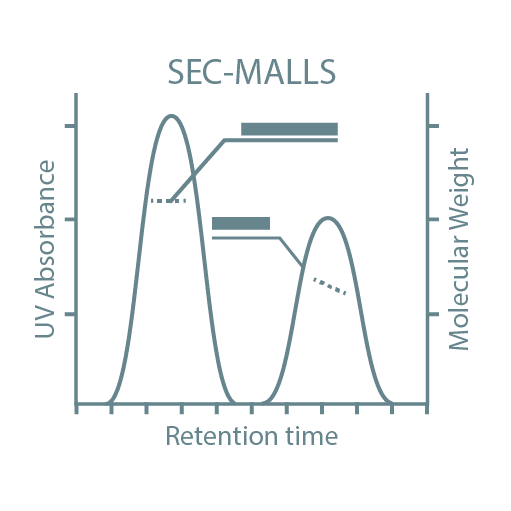
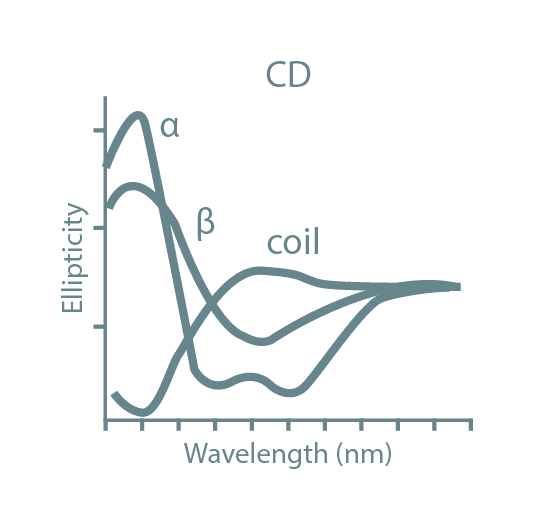
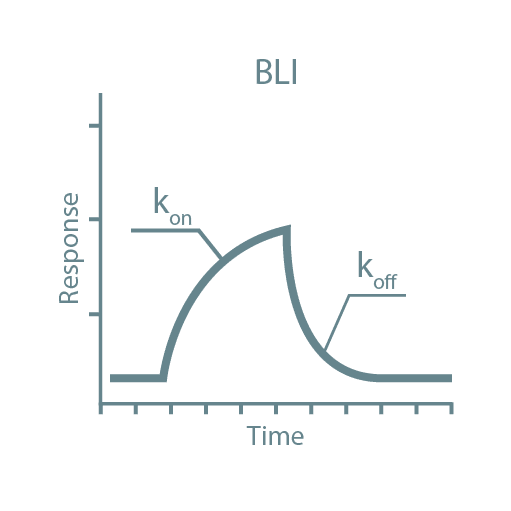
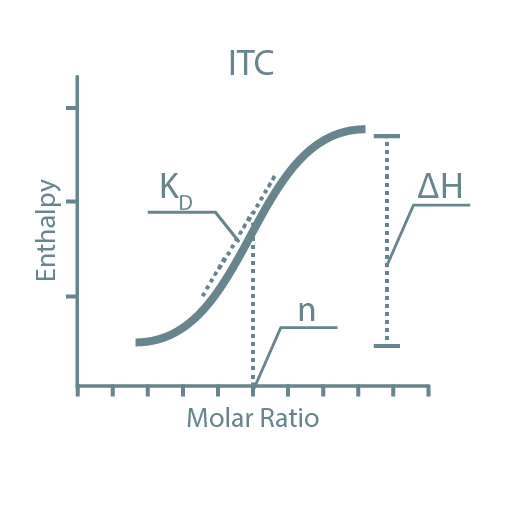
Following high-throughput protein screening, additional experiments can be performed to further characterize each protein sample. Typical characteristics analyzed include stability, activity, oligomeric state, secondary structure, protein-ligand affinity, and binding kinetics. For this, we utilize specialized instruments such as Thermal Shift Assays (TSA), Size-Exclusion Chromatography coupled with Multi-Angle Light Scattering (SEC-MALLS), Bio-Layer Interferometry (BLI), Isothermal Titration Calorimetry (ITC), and Circular Dichroism (CD).
Our expertise in protein research and technology will help you gain key insights into the quality, structure, and function of your proteins. These insights can translate into new perspectives and solutions for various applications, including vaccine development, antibody/protein engineering, biopesticide development, and biosensor development.


If you have any questions or would like to read our blog posts, click on the buttons below.